GOOD NEWS, Delica is proud to announce that we have received U.S. FDA approval for Transcranial Doppler EMS-9M, starting the process of serving the US market.
EMS-9M is the first wireless TCD in the world. It is a light, compact, and battery-armed system. It can be used for intracranial examinations, and examinations of peripheral and microvascular districts, both in sedentary and dynamic modes.
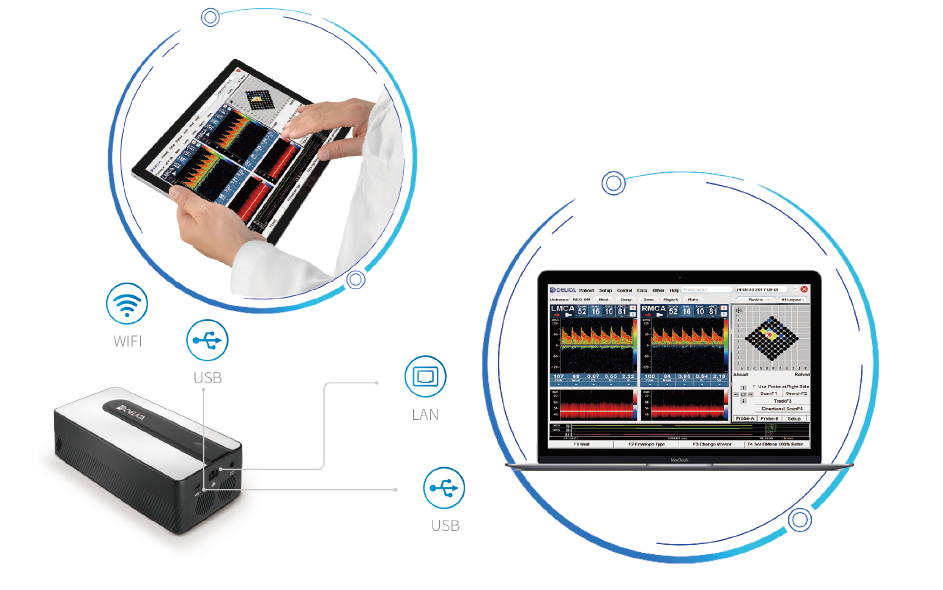
The Mini portable TCD have three data transmission designs LAN, USB, and WIFI, The patient's cerebral blood flow data can be easily transmitted to other monitoring system or ICM analysis platforms from the University of Cambridge in real-time. Build a long-term multimodal synchronous monitoring in a limited ICU space.
Uninterrupted monitoring is crucial to the safety of stroke patients during an emergency. Delica's EMS-9M TCD could proceed with the vascular evaluation and continuous monitoring of CBFV earlier while the patients are in emergency transit.
Additionally, Delica EMS-9M is a CE and NMPA-approved medical device that is widely used in clinical applications in Europe and China. They love this model with a good reputation.
 Delica Distributor Meeting in AmsterdamDelica Medical organized an international distributor meeting in Amsterdam, the Netherlands from April 2nd to 3rd. Nearly 28 participants from Europe and some other countries took part in this meeting.
Delica Distributor Meeting in AmsterdamDelica Medical organized an international distributor meeting in Amsterdam, the Netherlands from April 2nd to 3rd. Nearly 28 participants from Europe and some other countries took part in this meeting. Delica in Medica 2024From November 11 to 14, 2024 Dusseldorf International Medical and Medical Device Fair (MEDICA) was held in Dusseldorf Exhibition Center, Germany.
Delica in Medica 2024From November 11 to 14, 2024 Dusseldorf International Medical and Medical Device Fair (MEDICA) was held in Dusseldorf Exhibition Center, Germany. ENSCH 2025Delica comapny participated in ENSCH 2025 with High-End TCD products
ENSCH 2025Delica comapny participated in ENSCH 2025 with High-End TCD products ESOC 2025
ESOC 2025 DELICA made a wonderful appearance in MEDICAFrom November 13 to 16, 2023 Dusseldorf International Medical and Medical Device Fair (MEDICA) was held in Dusseldorf Exhibition Center, Germany.
DELICA made a wonderful appearance in MEDICAFrom November 13 to 16, 2023 Dusseldorf International Medical and Medical Device Fair (MEDICA) was held in Dusseldorf Exhibition Center, Germany. DELICA made a wonderful appearance in the 87th CMEFFrom May 14 to 17, 2023, the 87th China International Medical Equipment Fair (CMEF) was grandly opened in Shanghai National Convention and Exhibition Center. Nearly 5,000 elite enterprises gathered in the event, covering more than 320,000 square meters, nearly 100 conferences and forums, hundreds of special activities, and tens of thousands of products. Industry celebrities and elite gathered in the scene, innovative technology and cutting-edge academic blend here, infinite business opportunitie
DELICA made a wonderful appearance in the 87th CMEFFrom May 14 to 17, 2023, the 87th China International Medical Equipment Fair (CMEF) was grandly opened in Shanghai National Convention and Exhibition Center. Nearly 5,000 elite enterprises gathered in the event, covering more than 320,000 square meters, nearly 100 conferences and forums, hundreds of special activities, and tens of thousands of products. Industry celebrities and elite gathered in the scene, innovative technology and cutting-edge academic blend here, infinite business opportunitie 14th Zadar Summer School of Neurosonology and Stroke Management14th Zadar Summer School of Neurosonolgy and Stroke Management from the 23rd – 25th of June which will be organised as a live event.
14th Zadar Summer School of Neurosonology and Stroke Management14th Zadar Summer School of Neurosonolgy and Stroke Management from the 23rd – 25th of June which will be organised as a live event. Delica Showed Innovative Neuro Medical Products at Medica 2022Delica Showed Innovative Neuro Medical Products at Medica 2022
Delica Showed Innovative Neuro Medical Products at Medica 2022Delica Showed Innovative Neuro Medical Products at Medica 2022 Delica Medical Workshop at Haga Teaching hospitalDelica Workshop at Haga Teaching hospital. Thank you Dr Ruud Keunen for the wonderful lectures!
Delica Medical Workshop at Haga Teaching hospitalDelica Workshop at Haga Teaching hospital. Thank you Dr Ruud Keunen for the wonderful lectures! ENSCH conference came to a successful conclusionThe ENSCH conference came to a successful conclusion Very positive feedback was received from professors about Delica EDS system
ENSCH conference came to a successful conclusionThe ENSCH conference came to a successful conclusion Very positive feedback was received from professors about Delica EDS system